CONTAMINATED SITE
Isotope Tracer Technologies Europe (IT2E) offers a range of isotope analysis services to aid in site assessment, including applications for site monitoring, characterization, and developing remediation strategies. Stable isotope analysis can allow for comprehensive contaminant studies, providing information that can be used to:
- distinguish between sources of contaminants;
- assess the movements of contaminants;
- examine the physical processes that alter the amounts, and forms, of various contaminants;
- determine the degree to which remediation efforts are altering contaminants of interest.
The range of isotope analyses offered at IT2E, including the compound-specific stable isotope methods (CSIA), can provide detailed data that will provide decision-makers with the tools to make informed site assessment solutions. The advantages provided by isotope analysis for assessing the fate and sources of contaminants can be increased by combining the analyses of multiple isotopes within a single molecule. For example, using δ2H, δ13C & δ37Cl analysis in studies of chlorinated solvents, or using δ18O and δ15N for examining nitrate contamination. Multi-isotope approaches greatly improve upon the ability of traditional isotope analysis for source apportionment, by providing an additional dimension for source separation. These approaches increase the sensitivity of isotope analysis for source signature determination and pathway identification.
Isotope analyses can define a crucial step throughout the entire site assessment and remediation process. It can be used to gain information on a compound’s origin, sources, degradation, and pathways, obtaining better conceptual model and risk analysis assessment. Particularly, CSIA is useful in a wide range of applications, e.g. feasibility studies and optimization of bioremediation actions, natural attenuation, and evaluation of remediation systems.
What are the Benefits of CSIA?
CSIA can be used to answer several questions vital for informed decision-making throughout the entire site assessment and remediation process.
This is true from the early stages of site characterization, where CSIA should be carried out, in conjunction with the analysis of contaminant concentration, to answer the following questions:
- Are natural attenuation and biodegradation occurring?
- Since sites are rarely homogenous, which specific locations show signs of biological or abiotic degradation?
- To what extent, and under what conditions, will these natural processes degrade the contaminate of interest?
- What do these natural biodegradation pathways look like, and what by-products can we expect to form? Are these by-products potential contaminants as well?
- Are there multiple sources contributing to the contaminant pool?
During remedy selection, CSIA can be used to determine the following:
- Can monitored natural attenuation (MNA) be used to reduce contaminant concentration, in a feasible time period?
- In light of any natural attenuation that may be occurring, to what degree are remedial actions required?
- Are observed contaminant concentration changes caused by remediation efforts successfully reducing contaminant mass, or through simple dilution or other physical processes (i.e. sorption, diffusion)?
- What are the degradation pathways occurring for our contaminant of interest?
Once a remediation strategy has been selected, continued CSIA analysis should be undertaken at the chosen site locations throughout site monitoring. CSIA will enable site managers to determine:
- Is the chosen remediation strategy working to reduce the mass of the contaminating compound?
- At which sites are remediation efforts most successful?
- Are additional remediation efforts required to further reduce the mass of the contaminating compounds?
Figure 1 shows dual isotope fractionations during aerobic biodegradation of 1,2-dichloroethane (1,2-DCA), which was investigated by Palau et al. (2014) in order to illustrate the potential to use CSIA for degradation pathway delineation.
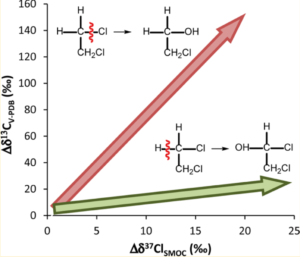
Figure 1: Dual isotope fractionations during oxidation and SN2 reaction of 1,2-dichloroethane (1,2-DCA), as investigated by Palau et al. (2014).

